The Light of Life——Red Light

一、Spectrum
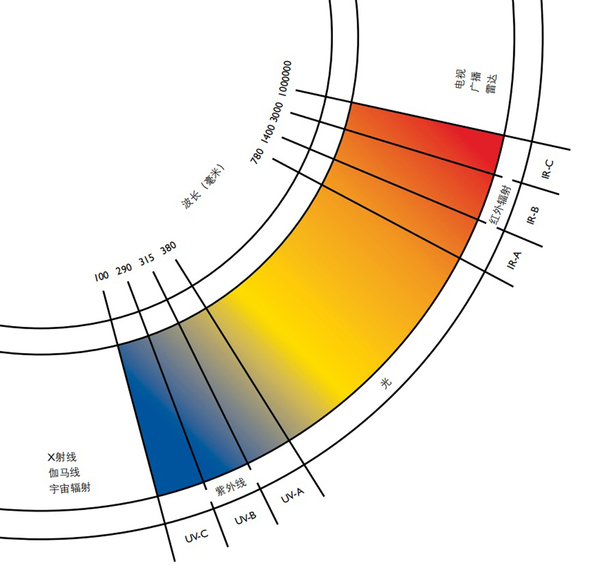
The light radiation spectrum is between 100 nanometers (ultraviolet range) and 1 mm (infrared range). For practical purposes, this wavelength range is divided into seven bands according to CIE (International Commission on Illumination).
100 to 280 nm UVC (short-wave ultraviolet)
280 to 315 nm UVB (medium wave ultraviolet)
315 to 380 (400) nanometer UVA (long wave ultraviolet)
Light from 380 (400) to 780 nanometers (visible light)
IR-A (short wave infrared radiation) from 780 to 1400 nm
1.4 to 3 microns IR-B (medium wave infrared radiation)
IR-C (long wave infrared radiation) from 3 microns to 1 mm
。
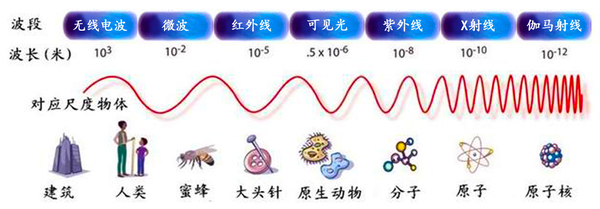
Wavelength Corresponds to Scale Objects
二、Photobiology, Photomedicine
Visible light, infrared radiation and ultraviolet light have many possible applications in photobiology and photomedicine. Visible light, infrared radiation and ultraviolet light have unique physical, photobiological and photochemical characteristics. From infrared to ultraviolet, the energy contained in light (light energy) gradually increases.
Most photobiological effects in the visible and ultraviolet ranges are produced by photochemical reactions, while the effects in the infrared radiation range are mainly due to heat dissipation.
三、Basic Rule
The photochemical reaction is controlled by several basic laws,The most important of these is: According to Grodius-Dreber's law, only when light is absorbed by the substance involved can the reaction between light and substance occur. If this is not the case, the radiation will be reflected, spread or dispersed.
The two laws are the Bunsen-Roscoe law or the law of reciprocity, that is, the number of reaction products of a photochemical reaction is proportional to the product of light irradiation and the irradiation time.This product is called a dose. In addition, in photobiology, the effect depends on the dose, not the intensity of the light. Irradiation with higher intensity for a shorter time or lower intensity for longer time can provide the same dose (same effect). Therefore, if "light" is absorbed by the skin, for example, the resulting effect depends on the radiation dose, not the radiation level. In photobiology, this is the so-called "dose-response" relationship of specific effects.
四、Photochemical Reaction, Photophysical Reaction
Red light acts on mitochondria to produce a series of biochemical reactions.
s mentioned earlier, light radiation can only take effect after being absorbed by the chromophore in the substance involved.This chromophore can be a biological molecule, such as DNA, RNA, protein, or drugs.
The absorbed visible light or ultraviolet can break or change the chemical chain in a single molecule, or establish a link between two or more molecules.
The absorbed infrared radiation can activate the rotation or vibration energy level in the molecule, causing a photophysical reaction. This absorption causes the heat in the absorbing material to dissipate.This warming effect is used in many medical applications, such as hyperthermia, hyperthermia and sports physiotherapy. However, it can also bring unnecessary side effects (depending on the wavelength).
五、Skin Structure
Skin Structure Diagram
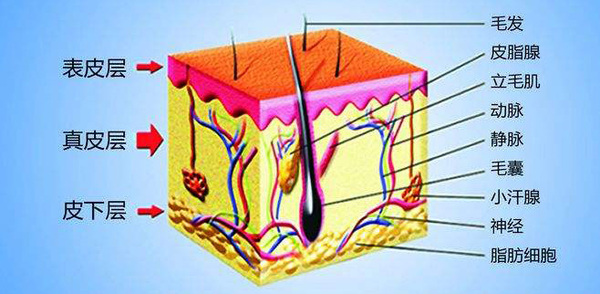
From a visual point of view, the skin can be regarded as an inhomogeneous medium, which consists of four layers:
-Stratum corneum-spine cortex-dermis (0.8-1 mm)-subcutaneous tissue (1-3 mm)
These layers have different refractive index and chromophore distribution, and will produce different reflection, propagation and scattering characteristics depending on the wavelength.
六、Skin "Optical Window
Schematic diagram of skin layering and wavelength penetration depth
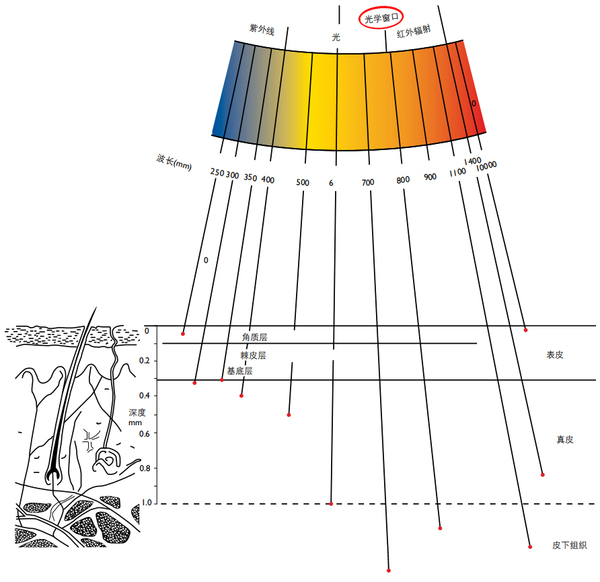
The attenuation of radiation in the epidermis is mainly due to the absorption of the chromophore, followed by scattering. The chromophores in the stratum corneum are mainly melanin, urocanic acid, and proteins composed of aromatic amino acids such as tyrosine and tryptophan. The epidermal germinal layer (= basal layer plus echinodermis) contains living cells (keratinocytes) and has the same chromophore as the stratum corneum, but here the nucleic acids of DNA and RNA play an important role in absorbing short-wave ultraviolet rays.
Due to the presence of blood vessels, the penetration of light into the dermis is also affected by the absorption of radiation by blood (hemoglobin and oxyhemoglobin) in the range of 400-600 nanometers and the scattering of collagen fibers. Most of the short-wave ultraviolet (UVC) is absorbed in the stratum corneum (90%), and 90% of the medium-wave ultraviolet (UVB) is absorbed in the epidermis, but a considerable part of the long-wave ultraviolet (UVA) can reach the dermal layer containing blood vessels . The thin epidermis has no blood vessels itself, but it can immediately receive the necessary substances from the capillaries under the basal cell layer of the epidermis.
Red Light, the "Optical Window" of the Skin
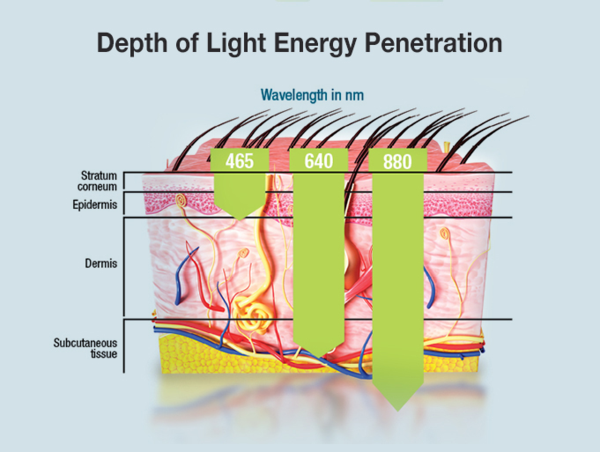
Red light with a wavelength of 600-940 nanometers can penetrate into the subcutaneous tissue, so it is called the "optical window" of the skin.